
Products
Original Factory Chondroitin 600mg - Ursodeoxycholic Acid(UDCA) of Deebio for Treating Gallstones – Deebiotech
Original Factory Chondroitin 600mg - Ursodeoxycholic Acid(UDCA) of Deebio for Treating Gallstones – Deebiotech Detail:
Detail
1. Characters: White powder; odorless. This product is soluble in ethanol, but insoluble in chloroform; soluble in glacial acetic acid, and soluble in sodium hydroxide test solution.
2. Process: Synthetic.
3. Indications and uses: For the treatment of gallstones, cholestatic liver disease, fatty liver, various types of hepatitis, toxic liver disorders, cholecystitis, cholecystitis and biliary dyspepsia, bile reflux gastritis, etc.
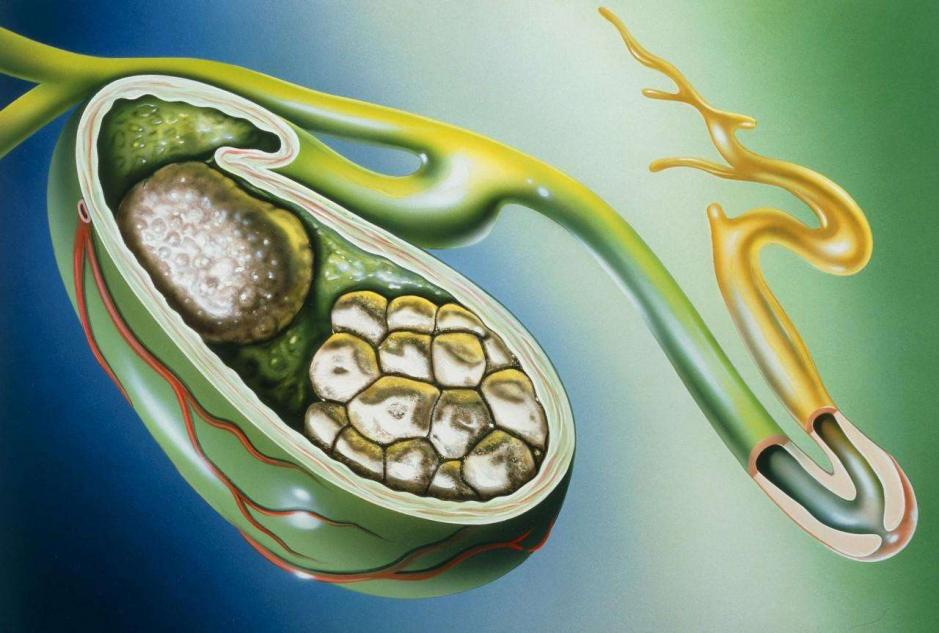
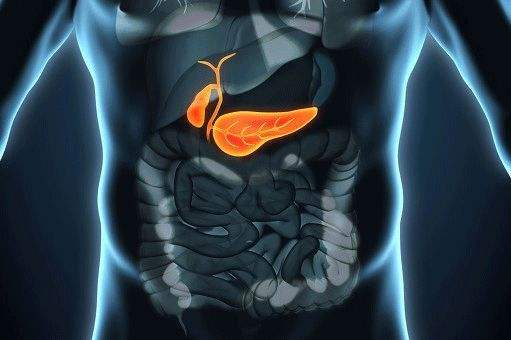
Why us?
·Produced in the GMP workshop
·27 years of biological enzyme R&D history
·Raw materials are traceable
·Comply with customer and enterprise standard
·Export to over 30 countries and regions
·Has the ability of quality system management such as US FDA, Japan PMDA, South Korea MFDS, etc.
Specification
|
Test Items |
According to internal standard and Customer Standard |
|
|
APPEARANCE |
White or almost white powder. |
|
|
MELTING RANGE |
Between 200°C and 205°C |
|
|
SPECIFIC ROTATION |
+57.0 〜+62.0° |
|
|
LOSS ON DRYING |
≤0.5% |
|
|
RESIDUE ON IGNITION |
≤0.1% |
|
|
ASSAY |
98.5%〜101.5% (Dried substance) |
|
|
PURITY (HPLC) |
≥98.5% |
|
|
RELATED SUBSTANCE (HPLC) |
≤1.5% (Chenodeoxycholicacid) |
Not detected |
|
≤0.1% (Unspecified impurities) |
0.05% |
|
|
≤0.1% (Lithocholicacid) |
Not detected |
|
|
≤1.5% (Total) |
0.10% |
|
|
RELATED SUBSTANCE (TLC) |
≤O.05% (Lithocholic acid) |
Complies |
|
≤1.5% (Chenodeoxycholicacid) |
Complies |
|
|
RESIDUAL SOLVENT |
Acetone: ≤5000ppm |
|
|
TOTAL AEROBIC MICROBIAL COUNT |
<103cfu/g |
|
|
TOTAL COMBINED YEAST/MOULDS COUNT |
<102cfu/g |
|
|
E.COLI |
Complies |
|
|
SALMONELLAE |
Complies |
|
|
CONCLUSION |
Qualified |
|
Product detail pictures:

Related Product Guide:
The business keeps to the operation concept "scientific management, premium quality and efficiency primacy, customer supreme for Original Factory Chondroitin 600mg - Ursodeoxycholic Acid(UDCA) of Deebio for Treating Gallstones – Deebiotech , The product will supply to all over the world, such as: Ukraine, Italy, Malaysia, we are fully determined to control the whole supply chain so as to provide quality products at competitive price in a timely manner. We are keeping up with the advanced techniques, growing through creating more values for our clients and society.
In our cooperated wholesalers, this company has the best quality and reasonable price, they are our first choice.

















